Phenolic Resins (Phenoplasts)
Properties and Synthesis
Phenolic resins (Phenoplasts) are synthetic thermosetting resins invented by Dr. Leo Baekeland in 1907. The material is often called Bakelite. Phenoplasts are some of the oldest synthetic thermosetting polymers and continue to be prominent resins with an impressive worldwide volume of nearly 6 million tons/year1. Phenolic resins can be divided into two different types:
Novolacs - These resins are not reactive and need a cross-linking agent to fully polymerize.
Resoles - These resins are self-curing due to the presence of reactive side groups
The phenolic resins are prepared by either acid-catalyzed (novolacs) or base-catalyzed (resoles) addition polymerization of phenol with formaldehyde. The resoles form in reactions of phenols with formaldehyde in water with catalytic amounts of a base (usually NaOH). The reaction is carried out at temperatures slightly above room temperature for several hours. A typical liquid resole resin has a rather low moelcular weight, and usually contains not more than two or three benzene rings. If the reaction is carried out a little further, it yields a solid resole. The pH is usually adjusted to neutral before the resin is crosslinked at elevated temperatures. The fusible resins are often called A-stage resins. Further condensation leads to a rubbery material called B-stage and the finally fully crosslinked resin is called C-stage resin.
Novolacs form at a pH below 7 by protonation of the carbonyl group of the formaldehyde followed by electrophilic aromatic substitution at the ortho or para positions of the phenol. The initial steps also take place in water, however, an excess of phenol has to be added to prevent the resin from crosslinking. The molecular weight of these resins depend on the phenol-formaldehyde ratio. The final crosslinking is achieved by addition of more formaldehyde or paraform, an oligomer of formaldehyde that decomposes to fomaldehyde.
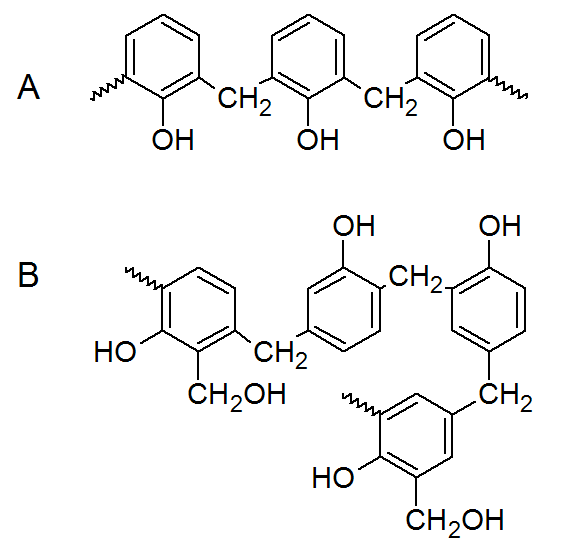
Both resole and novolak resins are important, high-volume, reactive intermediates that undergo a variety of chemical transformations. In many cases, the addition of other monomers leads to improved resin systems that add value to the resin products.
The phenolic resins are one of the most popular and versatile high-volume resin systems. They are fine machinable, lightweight and have excellent corrosion, and temperature resistance up to 300-350 °C. Furthermore, they have outstanding dimensional stability, high resistance to creep at elevated temperatures, low humidity absorption, high dielectric strength, and an excellent price/performance characteristic.
Commercial Phenolic Products
Major manufacturers of phenolic resins and phenolplast polymers are Arizona Chemicals (Sylvares™ Copolymers), Ashland (Arofene™), GP Chemicals (GP® and Leaf® resins), Hexion (Bakelite®), Mitsui Chemicals (MiLex™), and Quantum Composites (ESC® Phenolic).
Applications
Phenolic resins are used in coatings, composites, adhesives, and in molded parts. The phenolic materials are available in a variety of configurations, for example, with fiberglass and carbon fiber reinforcement or with various fillers and additive combinations, tailored to the requirements of specific industries and applications.
Besides being used in coatings for interiors of food and
beverage cans, phenolic resins are an excellent choice for
manufacturing protective coatings and for enhancing the
performance of epoxy, acrylic, polyester and alkyd based
adhesive or coating products. They are, for example, used for tank, drum, and pipe
linings; marine and industrial applications; and electrical
devices such as wires, motor and wound coils.
Due to
their low flammability, extremely low generation of toxic gases,
and slow heat release, they are often part of solutions for the
aerospace and transportation industry. For example, higher glass
or carbon fiber content grades are suitable for interior
structures of aircrafts. They are also used in products that are exposed to high-temperature and high-pressure
environments in industrial and oil and gas applications.
1Source: L. Pilato, Reactive and Functional Polymers, Vol. 73, Issue 2, p. 270 - 277 (2013)